Answer:
The remaining percentage of drug concentration is about 88.7% 2 years after manufacture.
Step-by-step explanation:
Recall the formula for the decay of a substance at an initial
 concentration at manufacture:
concentration at manufacture:
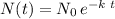
where k is the decay rate (in our case 0.06/year), and t is the elapsed time in years. Therefore, after 2 years since manufacture we have:
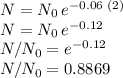
This in percent form is 88.7 %. That is, the remaining percentage of drug concentration is about 88.7% 2 years after manufacture.