Answer:
The final temperature of the bomb calorimeter is 82.98°C
Step-by-step explanation:
The given information are;
The mass of the propane sample = 10.0 g
The heat capacity of the bomb calorimeter = 8.0 kJ/°C
The molar heat of combustion of propane is -2,222 kJ/mol.
The starting (initial) temperature of the water, T₁ = 20°C
The final temperature of the bomb calorimeter = T₂
The molar mas of propane = 44.1 g/mol
The number of moles, n, of propane present in 10.0 g of propane is found as follows;
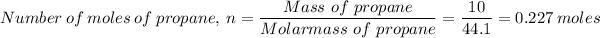 Which gives;
Which gives;
The heat, Δh, released from the combustion of 10.0 g of propane = 0.227 × -2222 kJ/mol
Δh = -503.85 kJ/mol
Heat gained by the calorimeter = Heat released from the combustion of 10.0 g of propane = 503.85 kJ/mol
Change in heat, Δh
 in the calorimeter = Heat capacity × Temperature change
in the calorimeter = Heat capacity × Temperature change
Δh
 = m × C
= m × C
503.85 kJ/mol = 8.0 kJ/°C × (T₂ - 20°C)
T₂ = -503.85 kJ/mol/(8.0 kJ/°C) + 20°C = 82.98°C
The final temperature of the bomb calorimeter = 82.98°C