Answer:
The new mass/volume percent is 2.0% (m/v)
Step-by-step explanation:
Dilution is a procedure by which the concentration of a solution is decreased, generally with the addition of a diluent. In other words, dilution is a process in which a concentrated solution is always started, to which a greater volume of solvent is added, causing the concentration and volume of the resulting solution to change. But the amount of solute used to prepare the initial solution remains the same.
The calculation of a dilution is made by:
Cinitial. Vinitial = Cfinal. Vfinal
where C indicates concentration and V indicates volume.
In this case:
- Cinitial: 6.0% (m/v)
- Vinitial: 110 mL
- Cfinal: ?
- Vfinal: 330 mL
Replacing:
6.0% (m/v) * 110 mL= Cfinal* 330 mL
Solving:
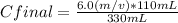
Cfinal= 2.0% (m/v)
The new mass/volume percent is 2.0% (m/v)