Answer:
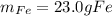
Step-by-step explanation:
Hello,
In this case, the undergoing chemical reaction is:
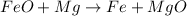
Thus, for the given masses of reactants we should compute the limiting reactant for which we first compute the available moles of iron (II) oxide:
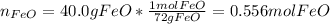
Next, we compute the consumed moles of iron (II) oxide by the 10.0 g of magnesium, considering their 1:1 molar ratio in the chemical reaction:
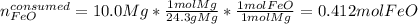
Therefore, we can notice there is less consumed iron (II) oxide than available for which it is in excess whereas magnesium is the limiting reactant. In such a way, the produced mass of iron turns out:
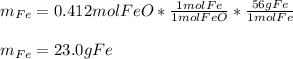
Regards.