Answer:
C.
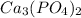 will precipitate out first
will precipitate out first
the percentage of
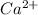 remaining = 12.86%
remaining = 12.86%
Step-by-step explanation:
Given that:
A solution contains:
![[Ca^(2+)] = 0.0440 \ M](https://img.qammunity.org/2021/formulas/chemistry/college/glpdf6z0lrfj2rs1e07fykkttcj2wfy79n.png)
![[Ag^+] = 0.0940 \ M](https://img.qammunity.org/2021/formulas/chemistry/college/i7io7xylcy07xcptrsj8aeb8ulsgkm7yqi.png)
From the list of options , Let find the dissociation of
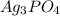
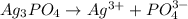
where;
Solubility product constant Ksp of
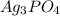 is
is
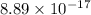
Thus;
![Ksp = [Ag^+]^3[PO_4^(3-)]](https://img.qammunity.org/2021/formulas/chemistry/college/dqsz6qyfx3sivyng5mlsfq050v4jd5i2xm.png)
replacing the known values in order to determine the unknown ; we have :
![8.89 * 10 ^(-17) = (0.0940)^3[PO_4^(3-)]](https://img.qammunity.org/2021/formulas/chemistry/college/w2qtrve6qq9l37dsln8wjdfvpm33h64mmc.png)
![(8.89 * 10 ^(-17))/((0.0940)^3) = [PO_4^(3-)]](https://img.qammunity.org/2021/formulas/chemistry/college/9fsc028cx3dqb05g2omhyykj1xvt9k9izw.png)
![[PO_4^(3-)] =(8.89 * 10 ^(-17))/((0.0940)^3)](https://img.qammunity.org/2021/formulas/chemistry/college/3xe91tq4f0ci4z2i34h5tnj0ug57txca9a.png)
![[PO_4^(3-)] =1.07 * 10^(-13)](https://img.qammunity.org/2021/formulas/chemistry/college/nm4y3k5272asgiyfgqtqotimw6avxaapql.png)
The dissociation of
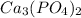
The solubility product constant of
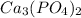 is
is
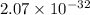
The dissociation of
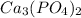 is :
is :
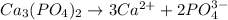
Thus;
![Ksp = [Ca^(2+)]^3 [PO_4^(3-)]^2](https://img.qammunity.org/2021/formulas/chemistry/college/lu9dtmunzfyea2qrxy30osc67ltw7engt5.png)
![2.07 * 10^(-33) = (0.0440)^3 [PO_4^(3-)]^2](https://img.qammunity.org/2021/formulas/chemistry/college/fq3qs2anomw02hxaz5uf413f4u2j4kjz7f.png)
![(2.07 * 10^(-33) )/((0.0440)^3)= [PO_4^(3-)]^2](https://img.qammunity.org/2021/formulas/chemistry/college/vszy2esotrsfsh1yhyykxxmzp4ufax7xta.png)
![[PO_4^(3-)]^2 = (2.07 * 10^(-33) )/((0.0440)^3)](https://img.qammunity.org/2021/formulas/chemistry/college/73zaitpspbdmv0ctlvk3ecu4cvp5p807hd.png)
![[PO_4^(3-)]^2 = 2.43 * 10^(-29)](https://img.qammunity.org/2021/formulas/chemistry/college/h47emmcmmhr3yyvehxj5ghqklfguht57lt.png)
![[PO_4^(3-)] = \sqrt{2.43 * 10^(-29)](https://img.qammunity.org/2021/formulas/chemistry/college/2s3fzqojgzwp1uqx3gy6x2xfp2q3n7ymjj.png)
![[PO_4^(3-)] =4.93 * 10^(-15)](https://img.qammunity.org/2021/formulas/chemistry/college/ucouty90iwfa3j3o6zmnqe8i37wbvwv80g.png)
Thus; the phosphate anion needed for precipitation is smaller i.e
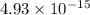 in
in
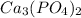 than in
than in
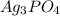
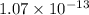
Therefore:
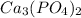 will precipitate out first
will precipitate out first
To determine the concentration of
![[Ca^+]](https://img.qammunity.org/2021/formulas/chemistry/college/dkqwiqt9e37inp40scyc7dbf1221gqshku.png) when the second cation starts to precipitate ; we have :
when the second cation starts to precipitate ; we have :
![Ksp = [Ca^(2+)]^3 [PO_4^(3-)]^2](https://img.qammunity.org/2021/formulas/chemistry/college/lu9dtmunzfyea2qrxy30osc67ltw7engt5.png)
![2.07 * 10^(-33) = [Ca^(2+)]^3 (1.07 * 10^(-13))^2](https://img.qammunity.org/2021/formulas/chemistry/college/uge2e1w1k5035r520ryhl36fhfnshoy7ju.png)
![[Ca^(2+)]^3 = (2.07 * 10^(-33) )/((1.07 * 10^(-13))^2)](https://img.qammunity.org/2021/formulas/chemistry/college/7qkdpqczu7elgmhz39nqnd6bgeu5pwdgjh.png)
![[Ca^(2+)]^3 =1.808 * 10^(-7)](https://img.qammunity.org/2021/formulas/chemistry/college/r7vy1jzdj2y69qgjx552khwhlkurfmksng.png)
![[Ca^(2+)] =\sqrt[3]{1.808 * 10^(-7)}](https://img.qammunity.org/2021/formulas/chemistry/college/tsg2f9un532jdjdu4lr8l191o19n5vywkv.png)
![[Ca^(2+)] =0.00566](https://img.qammunity.org/2021/formulas/chemistry/college/f07obchvt1s730ipq5hcqlhxmtvwmxjhnd.png)
This implies that when the second cation starts to precipitate ; the concentration of
![[Ca^(2+)]](https://img.qammunity.org/2021/formulas/chemistry/college/8qboeq70y9plosxl9jadhlt6p32n466poh.png) in the solution is 0.00566
in the solution is 0.00566
Therefore;
the percentage of
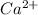 remaining = concentration remaining/initial concentration × 100%
remaining = concentration remaining/initial concentration × 100%
the percentage of
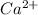 remaining = 0.00566/0.0440 × 100%
remaining = 0.00566/0.0440 × 100%
the percentage of
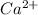 remaining = 0.1286 × 100%
remaining = 0.1286 × 100%
the percentage of
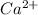 remaining = 12.86%
remaining = 12.86%