Answer:
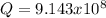
Yes, the precipitate of magnesium hydroxide will be formed.
Step-by-step explanation:
Hello,
In this case, the undergoing chemical reaction is:
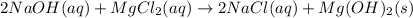
Whereas the precipitate is the magnesium hydroxide which is formed by:
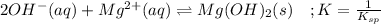
Take into account that the solubility product is the inverse reaction. In such a way, the equilibrium is:
![(1)/(K_(sp))=(1)/([OH^-]^2[Mg^(2+)])](https://img.qammunity.org/2021/formulas/chemistry/college/56bo96af21aut6cpkekvrmlhhylntre1db.png)
Thus, we know the concentration of OH⁻⁻ from the concentration of sodium hydroxide being the same (2.63x10⁻³M) since it is a strong base. Moreover, since magnesium chloride totally dissociates into magnesium and chloride ions the 1.80x10⁻³ M is also the concentration of magnesium ions.
Next, as the solutions mix, the final concentration of OH⁻⁻ is:
![[OH^-]=(75.0mL*2.63x10^(-3)M)/(75.0mL+125.0mL)=9.86x10^(-4)M](https://img.qammunity.org/2021/formulas/chemistry/college/6tvf5pswx6vaoebluc9one4i4ngplvktcs.png)
And the final concentration of Mg²⁺ is:
![[Mg^(2+)]=(125.0mL*1.80x10^(-3)M)/(75.0mL+125.0mL)=1.125x10^(-3)M](https://img.qammunity.org/2021/formulas/chemistry/college/7ei3uo5oftxuu9wi6d4y0jyi87grjr40l0.png)
Finally, we compute the reaction quotient:
![Q=(1)/([OH^-]^2[Mg^(2+)])=(1)/((9.86x10^(-4))^2*1.125x10^(-3)) =9.143x10^8](https://img.qammunity.org/2021/formulas/chemistry/college/mjb09jx6jezwiqdcwsz7tsos2eopbuvkxt.png)
But the equilibrium constant:
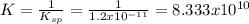
Therefore, since K>Q, we infer that the precipitate of magnesium hydroxide (consider the procedure) will be formed.
Regards.