Answer:
105.86 grams of AuCl3, if the compound has 6.3 x10^23 atoms of Cl.
Explanation:
We are given that the compound has 6.3 x10^23 atoms of Cl.
To find how many molecules of AuCl3 are in the given compound, we divide the compound by 3, i.e;
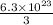 =
=
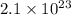 molecules of AuCl3.
molecules of AuCl3.
Now, as we know that 1 mole of AuCI3 has
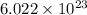 molecules.
molecules.
So, the moles that our compound has is given by;
=
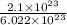 =
=
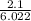 = 0.349 mole AuCI3
= 0.349 mole AuCI3
Also, the molar mass of AuCI3 = 303.33 g/mole
So, the molar mass of 0.349 moles AuCI3 =
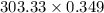
= 105.86 g
Hence, 105.86 grams of AuCl3, if the compound has 6.3 x10^23 atoms of Cl.