Answer:
The pressure cooker contains 0.000355 moles of air (to three significant figures).
Step-by-step explanation:
To find the number of moles of air in the pressure cooker, we can use the ideal gas law.
Ideal Gas Law

where:
- P is the pressure measured in pascal (Pa).
- V is the volume measured in cubic meters (m³).
- T is the temperature measured in kelvin (K)
- R is the ideal gas constant (8.3144626 J·K/mol).
- n is the number of moles.
Rearrange the equation to solve for n:
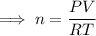
As we have been given the volume in liters, we must first convert it to cubic meters. As 1 liter = 0.001 m³ then 5.68 liters = 0.00568 m³.
Therefore, the values to substitute into the formula are:
- P = 205 Pa
- V = 0.00568 m³
- T = 394 K
- R = 8.3144626 J·K/mol
Substitute the values into the formula and solve for n:
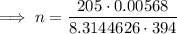
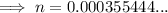
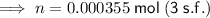
Therefore, the pressure cooker contains 0.000355 moles of air (to three significant figures).