Answer:
1.67 moles of iron (III) hydroxide are produced when 5.0 moles of sodium hydroxide reacts with excess iron (III) chloride.
Step-by-step explanation:
The balanced reaction is:
FeCl₃ + 3 NaOH ⇒ Fe(OH)₃+ 3 NaCl
By stoichiometry of the reaction (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of reagents and products participate in the reaction:
- FeCl₃: 1 mole
- NaOH: 3 moles
- Fe(OH)₃: 1 mole
- NaCl: 3 moles
Then you can apply the following rule of three: if 3 moles of NaOH form 1 mole of Fe(OH)₃, 5 moles of NaOH how many moles of Fe(OH)₃ do they form?
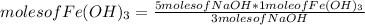
moles of Fe(OH)₃= 1.67
1.67 moles of iron (III) hydroxide are produced when 5.0 moles of sodium hydroxide reacts with excess iron (III) chloride.