Answer : The volume of HBr solution used should be, 0.125 L.
Explanation :
Formula used :

where,
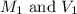 are the molarity and volume of HBr solution.
are the molarity and volume of HBr solution.
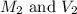 are the molarity and volume of diluted HBr solution.
are the molarity and volume of diluted HBr solution.
We are given:
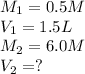
Putting values in above equation, we get:
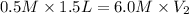
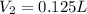
Therefore, the volume of HBr solution used should be, 0.125 L.