Answer:
See explanation.
Step-by-step explanation:
Hello,
In this case, for the described chemical reaction:
2 HCl(aq) + Mg(OH)2(aq) → MgCl2(aq) + 2 H2O(l)
We can notice there is a 2:1 molar ratio between the moles of hydrochloric acid and magnesium hydroxide, therefore, at the equivalence point:

And in terms of volumes and concentrations we verify:
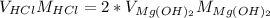
So we use the given data to proof it:
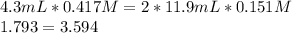
Therefore, we can conclude the data is wrong by means of the 2:1 mole ratio that for sure was not taken into account. This is also supported by the fact that normalities are actually the same, but the nomality of magnesium hydroxide is the half of the hydrochloric acid normality since the acid is monoprotic and the base has two hydroxyl ions.
Best regards.