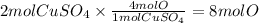Answer:
8 mol
Step-by-step explanation:
Step 1: Given data
Moles of copper (II) sulfate: 2 mol
Chemical formula of copper (II) sulfate: CuSO₄
Step 2: Establish the molar ratio of copper (II) sulfate to oxygen
According to the chemical formula, the molar ratio of copper (II) sulfate to oxygen is 1:4.
Step 3: Calculate the moles of O in 2 mol of CuSO₄