Answer:
The total photons required = 5.19 × 10²⁸ photons
Step-by-step explanation:
Given that:
the radiation wavelength λ= 12.5 cm = 0.125 m
Volume of the container = 0.250 L = 250 mL
The density of water = 1 g/mL
Density = mass /volume
Mass = Volume × Density
Thus; the mass of the water = 250 mL × 1 g/mL
the mass of the water = 250 g
the specific heat of water s = 4.18 J/g° C
the initial temperature
 = 20.0° C
= 20.0° C
the final temperature
 = 99° C
= 99° C
Change in temperature
 = (99-20)° C = 79 ° C
= (99-20)° C = 79 ° C
The heat q absorbed during the process = ms

The heat q absorbed during the process = 250 g × 4.18 J/g° C × 79° C
The heat q absorbed during the process = 82555 J
The energy of a photon can be represented by the equation :
= hc/λ
where;
h = planck's constant =
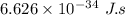
c = velocity of light =
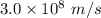
=
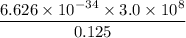
=
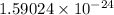 J
J
The total photons required = Total heat energy/ Energy of a photon
The total photons required =
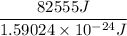
The total photons required = 5.19 × 10²⁸ photons