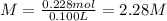Answer:
2.28 M
Step-by-step explanation:
Step 1: Given data
Percent by mass (%m/m): 20.0 %
Density (ρ): 1.114 g/mL
Step 2: Calculate the percent by volume (%m/v)
We will use the following expression.
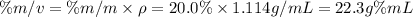
Step 3: Calculate the moles of solute in 100 mL of solution
The molar mass of phosphoric acid is 97.99 g/mol. The moles corresponding to 22.3 g are:
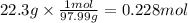
Step 4: Calculate the molarity of the solution
0.228 moles of solute are in 100 mL (0.100 L) of solution. The molarity of the solution is: