Answer:
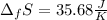
Step-by-step explanation:
Hello,
In this case, the entropy of fusion is computed in terms of the enthalpy of fusion considering the fusion temperature in kelvins:
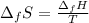
Thus, since the enthalpy of fusion is given in kJ/kg we must compute the grams of benzene in mole of benzene via its molar mass:
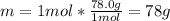
Next:
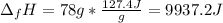
Finally, the entropy:
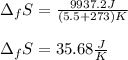
Best regards.