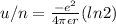Answer:
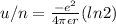
Step-by-step explanation:
given data
we will take here
sodium ions = positive charge
chloride ions = negative cgarge
solution
as when we take Na positive charge so Number of origin is
d = 0
and here pair of ions with negative charge at d = - r
and d = +r
therefore
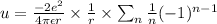
we will use here Taylor series approx method
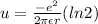
and N/2 pair will contribute here
so
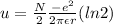
so energy per ion will be here