Answer:
The pH of the resulting solution is 9.02.
Step-by-step explanation:
The initial pH of the buffer solution can be found using the Henderson-Hasselbalch equation:
![pOH = pKb + log(([NH_(4)Cl])/([NH_(3)]))](https://img.qammunity.org/2021/formulas/chemistry/college/g6a4be1sltjxwyksd7rcsyp077riqz9jme.png)
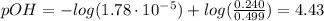
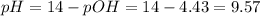
Now, the perchloric acid added will react with ammonia:
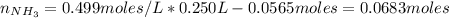
Also, the moles of ammonium chloride will increase in the same quantity according to the following reaction:
NH₃ + H₃O⁺ ⇄ NH₄⁺ + H₂O
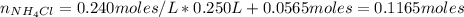
Finally, we can calculate the pH of the resulting solution:
![pOH = pKb + log(([NH_(4)Cl])/([NH_(3)]))](https://img.qammunity.org/2021/formulas/chemistry/college/g6a4be1sltjxwyksd7rcsyp077riqz9jme.png)
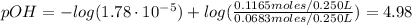
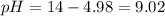
Therefore, the pH of the resulting solution is 9.02.
I hope it helps you!