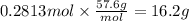Answer:
16.2 g
Step-by-step explanation:
Step 1: Write the balanced reaction
3 A + 2 B ⇒ 5 C
Step 2: Calculate the moles corresponding to 26.96 g of A
The molar mass of A is 159.7 g/mol.
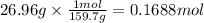
Step 3: Calculate the moles of C produced from 0.1688 moles of A
The molar ratio of A to C is 3:5. The moles of C produced are 5/3 × 0.1688 mol = 0.2813 mol.
Step 4: Calculate the mass corresponding to 0.2813 moles of C
The molar mass of C is 57.6 g/mol.