Answer:
The correct option is;
pH = 4.1
Explanation:
The given information are;
The hydrogen ion [H⁺] concentration in tomato juice =
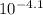
The pH of a solution = -㏒{H⁺] = -㏒(
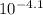 ) = -(-4.1) × ㏒(10) = 4.1
) = -(-4.1) × ㏒(10) = 4.1
The pH of the of the tomato juice = 4.1
The pH is the term used to refer to the acidity of water with the range of pH values ranging from 0 to 14 where pH values of a solution lesser than 7 are considered acidic while pH values above 7 indicate a basic solution.