Answer:
The essence including its given problem is outlined in the following segment on the context..
Step-by-step explanation:
The given values are:
Moles of CO₂,
x = 0.01962
Moles of water,
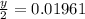
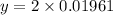
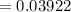
Compound's mass,
= 0.4647 g
Let the compound's formula will be:
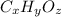
Combustion's general equation will be:
⇒
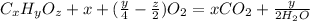
On putting the estimated values, we get
⇒
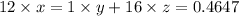
⇒
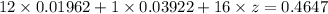
⇒
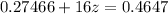
⇒
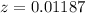
Now,
x : y : z =
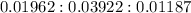
=
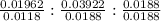
=
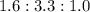
=
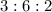
So that the empirical formula seems to be "C₃H₆O₂".