Answer:
The volume percent of graphite is 9.9%
Step-by-step explanation:
Given;
weight percent of graphite, C = 3.1wt%
density of ferrite,
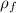 = 7.9 g/cm³
= 7.9 g/cm³
density of graphite,
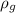 = 2.3 g/cm³
= 2.3 g/cm³
Determine the mass fraction;
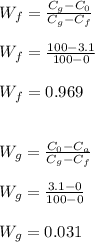
Determine the volume fraction;

Therefore, the volume percent of graphite is 9.9%