Answer:
See Explanaton
Step-by-step explanation:
The law of multiple proportions states that when two same elements form more than a compound, the different masses of one element that combine with the same mass of the other element are in the ratio of small whole numbers.
Oxide A
3.50g of sulphur combined with 6.00g of oxygen
Oxygen:Sulphur = 6 : 3.5
Oxide B
2.80g of sulphur combined with 9.55g
Oxygen : Sulphur = 9.55 : 2.8
Therefore:
The ratio of Oxygen to Sulphur in Oxides A and B is:
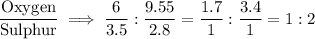
Ratio of Oxygen=1:2
There is exactly twice in Oxide B as in Oxide A.
This result illustrates the law of multiple proportions.