Answer:
267.14 iron oxide.
Step-by-step explanation:
Given that
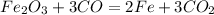
We know that molecular mass of iron-oxide = 160 g/mole.
We know that molecular mass of iron = 56 g/mole.
From the above reaction we can say that
1 mole of iron -oxide produce 2 mole of iron
160 gm of iron oxide produce 112 gm of iron
So
1 gm of iron required
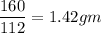 of iron oxide
of iron oxide
Therefore
187 gm of iron required
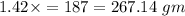 of iron oxide.
of iron oxide.
Therefore 187 gm of iron required 267.14 iron oxide.