Answer:
A. 0.35 M
Step-by-step explanation:
Hello,
In this case, given the volume and concentration of lithium hydroxide and the volume of chloric acid, we can compute the concentration of the neutralized acid by using the following equation:
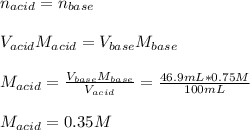
Therefore, answer is A. 0.35 M.
Regards.