Answer:
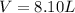
Step-by-step explanation:
Hello,
In this case, we can use the freezing point depression formula in order to compute the molality of the mentioned solute (ethylene glycol) considering a van't hoff factor of 1 since it is a covalent molecule:
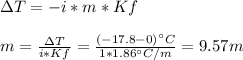
Next, since water density is 1 kg/L and molal units are mol/kg, we compute the present moles of solute:
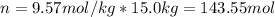
Next, the mass with its molar mass (62.07 g/mol):
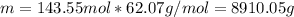
Finally, with the given density we compute the required volume in liters:
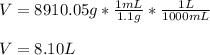
Best regards.