Answer: The solubility of gas increases in a solution if the pressure above the solution is increased
Step-by-step explanation:
Henry's law states that the amount of gas dissolved or molar solubility of gas is directly proportional to the partial pressure of the liquid.
To calculate the molar solubility, we use the equation given by Henry's law, which is:
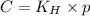
where,
C = solubility
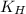 = Henry's constant
= Henry's constant
p = partial pressure
As the solubility is directly proportional to the pressure, thus increasing the pressure increases the solubility.