Answer:
4.123 × 10²³ molecules
Explanation:
To calculate the number of molecules first find the number of moles then multiply it by 6.02×10^23
number of moles(n) = mass / molar mass (M)
M of HCl = 1 + 35. 5 = 36.5g/mol
n = 25/36.5 = 0.6849mol
next we use the formula
N = n × L
N = number of entities
n = number of moles
L = Avogadro's constant 6.02 × 10^23 entities
Number of HCl molecules is
0.6849 × 6.02 × 10^23
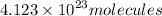
Hope this helps you