Answer:
503°C
Step-by-step explanation:
According to the given situation, the computation of the final temperature is shown below:
In this question we use the law of ideal gas i.e
pV = nRT
i.e

Therefore
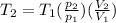
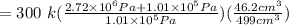
= 776 k
= (776 - 273)° C
= 503°C
Therefore the final temperature is 503°C
We simply applied the above formulas so that the final temperature could arrive