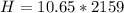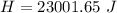Answer:
The heat is
Step-by-step explanation:
From the question we are told that
The inside temperature is
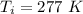
Te kitchen temperature is
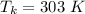
The workdone is
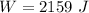
The coefficient of performance of the refrigerator is mathematically represented as
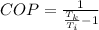
substituting values
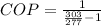
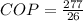
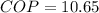
Now this coefficient of performance of the refrigerator can also be represented mathematically as
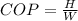
Where H is the heat which the refrigerator removes from the inside component
So
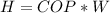
substituting values