Answer:
a.
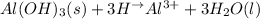
b.
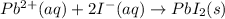
Step-by-step explanation:
Hello,
a. In this case, the overall reaction is:
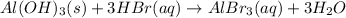
Nevertheless, the ionic version is:
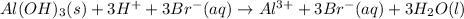
Since the base is insoluble, thereby, the balanced net ionic equation turns out:
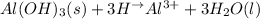
Since bromide ions become spectator ions.
b) In this case, the overall reaction is:
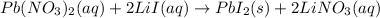
Nevertheless, the ionic version is:
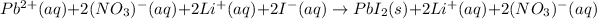
Since lead (II) iodide is insoluble whereas lithium nitrate does not, thereby, the net ionic equation turns out:
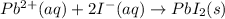
Since lithium and nitrate ions become spectator ions.
Regards.