Answer:
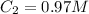
Step-by-step explanation:
Hello,
In this case, for dilution process, we can notice that the initial moles remain the same once the dilution is completed, therefore, both concentration and volume change considering:
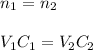
In such a way for the given final volume, the resulting concentration is noticed to be:
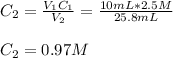
This is supported by the fact that the higher the volume the lower the concentration.
Best regards.