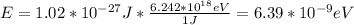Answer:
a. E = 1.02*10^-27 J
b. E = 6.39*10^-9eV
Step-by-step explanation:
a. In order to calculate the energy of the radio photon, you use the following formula:
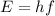 (1)
(1)
h: Planck's constant = 6.626*10^-34 Js
f: frequency of the photon = 1545kHz = 1.545*10^6 Hz
Then, by replacing you obtain the energy of the photon:
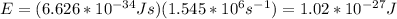
b. In electron volts, the energy of the photon is: