Answer:
(a)
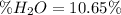
(b)
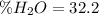
Step-by-step explanation:
Hello.
For this questions we must consider the ratio of the molar mass of water to hydrated compound molar mass as shown below:
(a) In this case, we can consider that inside the manganese (II) sulfate monohydrate, whose molar mass is 169.02 g/mol, there is one water molecule that has a molar mass of 18 g/mol, for which the theoretical percentage of water is:
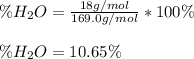
(b) In this case, we can consider that inside the manganese (II) sulfate tetrahydrate, whose molar mass is 223.1 g/mol, there are four water molecules that have a molar mass of 4*18 g/mol, for which the theoretical percentage of water is:
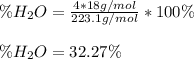
Best regards.