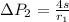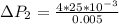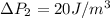Complete Question
The complete question is shown on the first uploaded image
Answer:
The pressure difference of the first bubble is
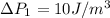
The pressure difference of the second bubble is
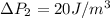
The pressure difference on the second bubble is higher than that of the first bubble so when the valve is opened pressure from second bubble will cause air to flow toward the first bubble making is bigger
Step-by-step explanation:
From the question we are told that
The radius of the first bubble is
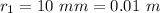
The radius of the second bubble is
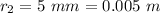
The surface tension of the soap solution is
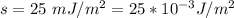
Generally according to the Laplace's Law for a spherical membrane the pressure difference is mathematically represented as
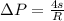
Now the pressure difference for the first bubble is mathematically evaluated as
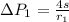
substituting values
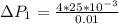
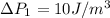
Now the pressure difference for the second bubble is mathematically evaluated as