Answer:
1. 2+ (
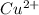 ).
).
2. 0 (
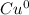 ).
).
Step-by-step explanation:
Hello,
In this case, the described chemical reaction is a redox reaction in fact, since the oxidation states of both magnesium and copper change as shown due to the displacement:
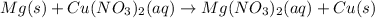
Therefore:
1. Since copper is the cation in the copper (II) nitrate, the (II) means that its charge is 2+ (
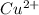 ).
).
2. Since copper is alone, it means no electrons are being neither shared not given, its charge is 0 (
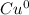 ).
).
Best regards.