Answer:
The limiting reactant between is NH₃.
Step-by-step explanation:
The reaction of the Solvay process is:
CO₂(g) + NH₃(g) + H₂O(l) + NaCl(s) ⇄ NaHCO₃(s) + NH₄Cl(aq) (1)
Since the water and the sodium chloride are in excess we need to find the number of moles of CO₂ and NH₃ at STP (1 amt, 273 K).
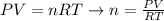
Where:
n: is the number of moles
P: is the pressure = 1 atm
V: is the volume
T: is the temperature = 273 K
R: is the gas constant = 0.082 L*atm(K*mol)
For CO₂ we have:
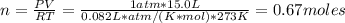
And for NH₃ we have:
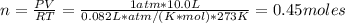
From the equation (1) we have that 1 mol of CO₂ reacts with 1 mol of NH₃, so from that ratio we have:
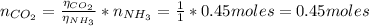
From above we have that 1 mol of NH₃ reacts with 0.45 moles of CO₂, and we have 0.67 moles of CO₂, hence the limiting reactant is NH₃.
Therefore, the limiting reactant between CO₂ and NH₃ is NH₃.
I hope it helps you!