Answer:
Molality = 1.428m
Step-by-step explanation:
Molality, m, is an unit of concentration defined as the ratio between moles of solute and kg of solvent:
Molality: Moles solute / kg solvent.
In the problem, water is the solvent (Is the compound in the higher quantity) whereas NaNO₃ is the solute.
Moles of solute: 0.3355moles NaNO₃.
Kg solvent:
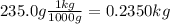
Thus, molality of the solution is:
Molality = 0.3355 moles NaNO₃ / 0.2350kg
Molality = 1.428m