Answer:
- Iron is oxidized from 0 to +2.
- Hydrogen is reduced from +1 to 0.
Step-by-step explanation:
Hello,
In this case, for the given reaction, we must first indicate the oxidation state of each element (notice that HC2H3O is acetic acid)
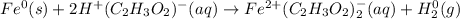
Thereby, notcing that iron is alone at the beginning, it has zero as its oxidation state, but once the reaction is undergone, it changes to +2, moreover, the displaced hydrogen in the acetic acid is with +1 but after the chemical reaction it becomes alone for which its oxidation state is 0, thereby, we find that:
- Iron is oxidized from 0 to +2.
- Hydrogen is reduced from +1 to 0.
Best regards,