Answer:
pH = 10.93
Step-by-step explanation:
To find the pH we need to use the Henderson - Hasselbalch equation:
![pH = pKa + log(([A^(-)])/([HA]))](https://img.qammunity.org/2021/formulas/chemistry/college/e9je4fdbiu8m4j3iueg4yk78w0dxcyf0qu.png)
Where:
[A⁻]: is the conjugate base of the acid = [CH₃NH₂] = 0.55 M
[HA]: is the acid = [CH₃NH₃Cl] = 0.29 M
Since the pkb of methylamine is 3.35, the pka is:
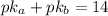
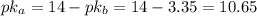
Now, the pH is:
![pH = pKa + log(([A^(-)])/([HA])) = 10.65 + log((0.55)/(0.29)) = 10.93](https://img.qammunity.org/2021/formulas/chemistry/college/q2i3yprer6wt25hoys7ln29xedak8s3xrp.png)
Therefore, the pH of the buffer solution is 10.93.
I hope it helps you!