Answer: B) 46.7% Si and 53.3% O
Step-by-step explanation:
To calculate the mass percent of element in a given compound, we use the formula:
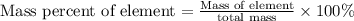
Mass of quartz
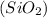 = 5.05 g
= 5.05 g
Mass of silicon = 2.36 g
Mass of oxygen = Mass of quartz
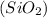 - mass of silicon = 5.05g - 2.36 g = 2.69 g
- mass of silicon = 5.05g - 2.36 g = 2.69 g
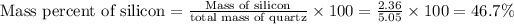
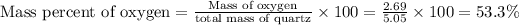
Thus the percentages of silicon and oxygen in quartz are B) 46.7% Si and 53.3% O