Answer:
Cellular respiration:
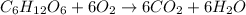
Step-by-step explanation:
Hello,
In this case, the law of conservation of mass states that matter cannot be created nor destroyed but modified, therefore, typical examples are the raft chemical reactions in nature or carried out in a laboratory. Thus, a typical one that could be daily found is in the cellular respiration wherein glucose and oxygen are converted into carbon dioxide, water and energy for us to use it in our daily activities. It is chemically represented by:
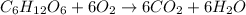
Law of conservation of mass is verified as six carbon atoms are before and after the chemical reaction, twelve hydrogen atoms are before and after it and eighteen oxygen atoms as well.
Best regards.