Answer:
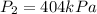
Step-by-step explanation:
Hello,
In this case, the Boyle's is mathematically defined via:

Which stands for an inversely proportional relationship between volume and pressure, it means the higher the volume the lower the pressure and vice versa. In such a way, since the volume is decreased to one quarter, we can write:
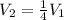
We can compute the new pressure:
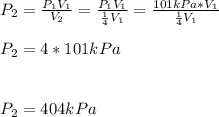
Which means the pressure is increased by a factor of four.
Regards.