Answer:
0.45 moles
Step-by-step explanation:
The computation of the number of moles left in the cylinder is shown below:
As we know that
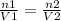
we can say that
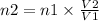
where,
n1 = 1.80 moles of gas
V2 = 12.0 L
And, the V1 = 48.0 L
Now placing these values to the above formula
So, the moles of gas in n2 left is
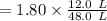
= 0.45 moles
We simply applied the above formulas so that the n2 moles of gas could arrive