Answer:
C. 101.5°C
Step-by-step explanation:
The computation of the boiling point of a solution is shown below:
As we know that
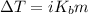
where,
i = Factor of Van’t Hoff i.e 2KBr
K_b = Boiling point i.e 0.512° C
m = molality
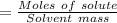
Now place the values to the above formula
So, the boiling point is
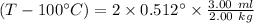
After solving this, the t is
= 100° C + 1.536° C
= 101.536° C
Hence, the correct option is C.