Answer:
Vapour pressure of the solution is 223.8mmHg
Step-by-step explanation:
Based on Raoult's law, vapour pressure of a solution decreases related to the vapour pressure of the pure solvent following the equation:
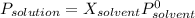
Where P is vapour pressure and X mole fraction of each related substance.
Vapour pressure of pure water is 233.8mmHg
To find Mole fraction of the solution you need to find moles of glycerin and moles of water, mole fraction of water will be:
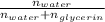
Moles of water (Molar mass: 18.02g/mol):
125g × (1mol / 18.02g) = 6.937 moles water
Moles of glycerin (Molar mass: 92.09g/mol):
28.5g × (1mol / 92.09g/mol) = 0.309 moles glycerin
Thus, mole fraction of water, X, is:
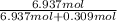
= 0.957
Replacing in Raoult's law:
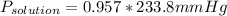
P = 223.8mm Hg
Vapour pressure of the solution is 223.8mmHg