Answer:
% purity of limestone = 96.53%
Step-by-step explanation:
Question (4).
Weight of impure CaCO₃ = 25.9 g
Molecular weight of CaCO₃ = 40 + 12 + 3(16)
= 100 g per mole
We know at S.T.P. number of moles of CO₂ = 1 and volume = 22.4 liters
From the given reaction, 1 mole of CaCO₃ reacts with 1 mole or 22.4 liters of
CO₂.
∵ 22.4 liters of CO₂ was produced from CaCO3 = 100 g
∴ 1 liter of CO₂ will be produced by CaCO₃ =
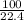
∴ 5.6 liters of CO₂ will be produced by CaCO₃ =
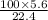
= 25 g
Therefore, % purity of CaCO₃ =
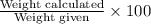
=

= 96.53 %