Answer:
final volume V2 = 0.71136 m³
work done in process W = -291.24 kJ
heat transfer Q = 164 kJ
Step-by-step explanation:
given data
mass = 1.5 kg
pressure p1 = 200 kPa
temperature t1 = 150°C
final pressure p2 = 600 kPa
final temperature t2 = 350°C
solution
we will use here superheated water table that is
for pressure 200 kPa and 150°C temperature
v1 = 0.95964 m³/kg
u1 = 2576.87 kJ/kg
and
for pressure 600 kPa and 350°C temperature
v2 = 0.47424 m³/kg
u2 = 2881.12 kJ/kg
so v1 is express as
V1 = v1 × m ............................1
V1 = 0.95964 × 1.5
V1 = 1.43946 m³
and
V2 = v2 × m ............................2
V2 = 0.47424 × 1.5
final volume V2 = 0.71136 m³
and
W = P(avg) × dV .............................3
P(avg) =
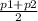 =
=
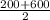 = 400 × 10³
= 400 × 10³
put here value
W = 400 × 10³ × (0.71136 - 1.43946 )
work done in process W = -291.24 kJ
and
heat transfer is
Q = m × (u2 - u1) + W .............................4
Q = 1.5 × (2881.12 - 2576.87) + 292.24
heat transfer Q = 164 kJ