Answer: The mass percent by volume is 101.6%
Step-by-step explanation:
The solution concentration expressed in percent by volume means that the amount of solute present in 100 parts volume of solution.
It is represented in formula as :
mass percent by volume =
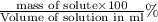
Given : mass of glucose = 330.1 g
volume of solution = 325 ml
Thus mass percent by volume =
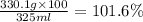
Thus the mass percent by volume is 101.6%