Answer: molar ratio: 0.58
Step-by-step explanation:
This is a buffer problem. We would use the equation
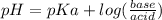 . Since we were given Ka and pH, we can find the ratio.
. Since we were given Ka and pH, we can find the ratio.
pKa=-log(Ka)
pKa=-log(1.8×10⁻⁵)
pKa=4.74
Now that we have pKa, we can plug in our values and find the molar ratio. Instead of writing base/acid, let's use x.
4.5=4.74+log(x)
-0.24=log(x)
10⁻⁰⁻²⁴=x
x=0.58