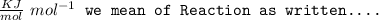Answer:
The answer is "−847 J/K".
Step-by-step explanation:
The given expression is:
2Al(s)+ Fe2O3(s) → Al2O3(s)+ 2Fe(s)
Δ
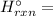 ∑(Δ
∑(Δ
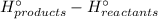 )
)
by the above definition Δ
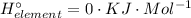 For Such a Component under standard conditions from its standard state, that also applies here. But, we start taking the overview and follow the conventions of signing:
For Such a Component under standard conditions from its standard state, that also applies here. But, we start taking the overview and follow the conventions of signing:
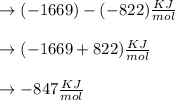
Δ
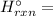 -847
-847